Press and Media
TINNITUS TOGETHER
Beginning January 15, 2024, our collaborators at the Hough Ear Institute (HEI) will be offering Tinnitus Together, the first peer-to-peer tinnitus support group in the State of Oklahoma. This no-cost support group will be led by a licensed professional counselor, Caleb Scoville, at Crossings Community Church on the 1st and 3rd Monday of every month. This project is intended to bridge the gap between the present need for support and a future in which medications become available. For more information https://www.houghear.org/tinnitus-together
Boehringer OKs $100M biobucks deal for hearing loss R&D project
Response to OKN-007 and NAC in a Patient with UnilateralHearing Loss and Chronic Tinnitus from VestibularSchwannoma
Enhanced sucrose-mediated cryoprotection of siRNA-loaded poly (lactic-co-glycolic acid) nanoparticles
2020 – 2021 Updates from Otologic Pharaceutics
Articles about us:
- Oblato Acquires Rights to Hearing Loss Drug (https://hearingreview.com/inside-hearing/research/hei)
- Bringing Back Silence (https://houghear.org/bringing-back-silence/)
Noteworthy News:
- Our President, Dr. Elaine Hamm, was an invited speaker to the first annual Inner Ear Disorders Therapeutic Summit in 2021. She spoke about the challenges of drug development for startups, particularly in the Midwest. https://innerear-disorders-therapeutics.com/whats-on/speakers/
- Our team was the first, to our knowledge, to restore the function of outer hair cells in the cochlea. The esteemed journal, Hearing Research, published this major revelation.
- Our researchers found a second molecule (HEI-115) makes AOK-1 even more effective. When used together, they boost hearing recovery!
- The our collaborators at Hough Ear Institute discovered our hearing loss pill (NHPN-1010) may treat tinnitus. The leading, peer-reviewed journal, PLOS One, published these breakthrough data.
- Finally, in 2020 our collaborators at Hough received a large OCAST grant. It funded the start of a proof-of-concept study for the treatment of tinnitus. Data from this study could lead to an FDA Phase II clinical trial for treating tinnitus!
Electrophysiological assessment and pharmacological treatment of blast-induced tinnitus
Potential Hearing Loss Drug Featured in “The Scientist”
FOR IMMEDIATE RELEASE
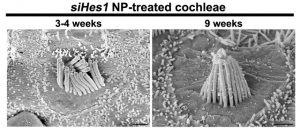
At both three and nine weeks after guinea pigs’ cochleae were treated with nanoparticles loaded with Hes1 siRNA, the authors observed what are likely immature hair cells. MODIFIED FROM X. DU ET AL., MOLECULAR THERAPY, 2018
Potential Hearing Loss Drug Featured in The Scientist
- Otologic Pharmaceutics, in collaboration with the Hough Ear Institute, has developed a new drug that could restore hearing
- Through siRNA therapy, OPI/HEI scientists are able to regrow lost or damaged hair cells in the ear
OKLAHOMA CITY, Okla., April 30th 2018. On April 18th The Scientist, a magazine for life science professionals, published an article focusing on the success of The Hough Ear Institute (HEI) and Otologic Pharmaceutics Inc. (OPI) in their recent efforts to restore hearing. The preclinical study uses small interfering RNAs to initiate the regeneration of hair cells in the cochlea of noise deafened adult guinea pigs and was published in Molecular Therapy.
“The loss of hair cells in the cochlear of the ear results in deafness. Unfortunately, because hair cells are unable to regrow, loss or damage to these cells is permanent, as is the hearing loss that it causes.” said Richard Gammans Ph.D., CEO of OPI. “Hearing aids can make sounds louder but they really only work when there are at least some remaining hair cells. With our therapy, we can actually trigger the cells in the cochlear to regenerate and grow to functional hair cells. Dr. Gammans also added “We believe this can be done without having to undergo a complicated surgery that requires direct injection into the cochlear. Although we are in the early stages, this therapy has the potential to dramatically alter the lives of patients who have lost their hearing from a traumatic injury, excessive noise, or even ageing.”
Dr. Elaine Hamm, COO of Otologic Pharmaceutics, also stated, “Our team at HEI and OPI is committed to developing innovative therapies that will impact how people live. Curing deafness is one such area. To be a part of such a transformative drug development program is incredibly exciting.”
OPI and HEI’s next steps with this innovative technology will focus on drug formulation and efficient drug delivery. OPI and HEI recently received an almost $2 million grant from the Department of Defense to pursue these studies.
About Otologic Pharmaceutics, Inc
OPI’s exploration of drugs for the treatment of hearing and balance disorders has led to product candidates aimed at both chronic and acute hearing loss. Located in Oklahoma City, Oklahoma, OPI has received local investment from i2E Inc., the Presbyterian Health Foundation, Accele BioPharma, and local Oklahoma Angel investors among others. OPI has the worldwide exclusive license to the hair cell regeneration technology as well as the license for another, later stage therapy, NHPN-1010. OPI has also successfully completed a Phase 1 clinical trial for the product NHPN-1010, a treatment for acute Noise-Induced Hearing Loss (NIHL), tinnitus, and balance disorders, like vertigo and Meniere’s Disease.
About The Hough Ear Institute
HEI is a non-profit 501c3 organization dedicated to improving hearing and balance of people worldwide. Their mission is to restore hearing worldwide through research, education, and humanitarian efforts. Scientific research with the aim of improving the hearing of those with acquired hearing loss is a major thrust of the Institute’s endeavors.
For more information, contact Dr. Elaine Hamm, at ehamm at accelebio dot com
The Scientist: https://www.the-scientist.com/?articles.view/articleNo/52319/title/RNA-Injection-Restores-Hearing-in-Guinea-Pigs/
Molecular Therapy: https://www.cell.com/molecular-therapy-family/molecular-therapy/fulltext/S1525-0016(18)30112-6
https://authors.elsevier.com/a/1W~MB5QliRlQ36
Otologic Pharmaceutics and Hough Ear Institute Awarded $1.9 million Grant to Advance Cochlear Hair Cell Regeneration Treatment
Only Funded Application Among 73 Applicants
Otologic Pharmaceutics, Inc. (OPI), in alliance with The Hough Ear Institute (HEI), announces the award of a Congressionally Directed Medical Research Programs (CDMRP) grant from the Department of Defense. The grant of $1.9 million will be used to further the preclinical research and development of an innovative therapy for the loss of hearing and equilibrium caused by a demise of sensory hair cells in the inner ear. OPI and HEI received the only funded award from among 73 applications in the Hearing Research Program.
The therapy, which was initially developed by HEI, uses small molecules and interfering nucleic acids to affect the regeneration of sensory hair cells within the cochlea. Trauma, ageing, infection, and toxins are some of the factors that can contribute to a loss of sensory hair cells. A reduction in sensory hair cells has a detrimental effect on hearing and equilibrium and, once lost, hair cells do not spontaneously regenerate, creating a permanent impairment. In addition to aiding those who suffer from acute hearing and balance impairments stemming from sensory hair cell injury, this therapy has the potential to impact the tens of millions of people worldwide who live with chronic, age-related hearing loss (presbycusis), for which there are no approved treatments.
The CDMRP funds will be used to complete the preclinical trial testing and characterization of this innovative therapy.
“We are very pleased to collaborate with HEI on this grant,” said Richard Gammans, Ph.D., CEO of OPI. “These funds allow us to advance the characterization of the treatment and advance it towards human studies.”
About Hearing Loss
Hearing impairment is an exceedingly common health condition, affecting millions of people worldwide. The World Health Organization (WHO) estimates that more than 360 million individuals worldwide suffer from disabling hearing loss and the National Institutes of Health (NIH) estimates that 36 million Americans have some degree of hearing loss. NIH also estimates that one in three people between the ages of 65 and 74 in the United States has hearing loss, a substantial portion of which goes untreated. According to the Department of Defense and the Department of Veterans Affairs, the two most frequent service-connected disabilities in the military are related to hearing disorders, with 1.1 million veterans living with hearing loss. Damage – by trauma, ageing, toxins, or other factors – to sensory hair cells or to auditory nerve cells accounts for an estimated 90 percent of hearing loss. Sensory hair cells do not spontaneously regenerate, causing a permanent loss of hearing. Hearing loss costs the US up to $56 billion per year in lost productivity, retraining, and health care for the hard of hearing.
About Otologic Pharmaceutics, Inc.
OPI’s exploration of innovative therapies to treat hearing and balance disorders has led to product candidates aimed at both chronic and acute hearing loss. The loss of sensory hair cells commonly causes loss in both hearing and equilibrium. Preclinical testing in the use of small molecules and interfering nucleic acids to regenerate sensory hair cells has led to a CDMRP grant to further the research and development of this promising technology. OPI has also successfully completed a single and multi-dose Phase I clinical trial for the product NHPN-1010, a treatment for acute Noise Induced Hearing Loss (NIHL) and tinnitus. Preparations have begun for a Phase II clinical trial of NHPN-1010 in patients with hearing disorders.
About The Hough Ear Institute
HEI is a non-profit 501c3 organization dedicated to improving hearing and balance of people globally. Their mission is to restore hearing worldwide through research, education, and humanitarian efforts. Scientific research, with the aim of improving the hearing of those with acquired hearing loss, is a major thrust of the Institute’s endeavors.
“We are excited and grateful to have received this very competitive grant award,” said Richard D. Kopke, MD, FACS, CEO of Hough Ear Institute. “Our research team is thrilled to advance a treatment that restores hearing to patients.”
Otologic Pharmaceutics, Inc. successfully completes Phase I trial to treat Noise Induced Hearing Loss
Otologic Pharmaceutics, Inc. (OPI), a development-stage biopharmaceutical company committed to developing and commercializing novel pharmacological solutions and approaches to treat hearing disorders, announced the successful completion of single and multi-dose Phase I studies for its lead product, NHPN-1010, in healthy volunteers.
NHPN-1010 is an oral, fixed dose combination of two antioxidant molecules, HPN-o7 and n-acetylcysteine (NAC). The drug is designed for the treatment of Noise Induced Hearing Loss (NIHL). NHPN-1010 was developed in partnership with The Hough Ear Institute (HEI), a non-profit 501c3 organization aimed at the discovery of innovative technologies to improve hearing and balance.
“We are enthusiastic about the positive safety and pharmacokinetic results of the single and multi-dose Phase I trials,” said Richard Gammans, Ph.D., COO of OPI. “This product has the potential to positively impact the lives of millions of people worldwide. The sext step is to plan for a Phase II trial of NHPN-1010.”
NHPN-1010 was examined in a randomized, double-blind, placebo-controlled Phase I study for safety, tolerability, and pharmacokinetics in single and multi-dose administrations. The initial aspect of the study used a single ascending dose method to determine the maximum tolerated dose (MTD) of HPN-07, as well as examining the MTD of HPN-07 in combination with a single fixed dose of NAC. All 32 of the subjects tested tolerated the drug well, with all subjects completing the study protocol. Following this, NHPN-1010 was evaluated in both enteric coated and uncoated forms in a fixed dose over 14 consecutive days. All 20 of the subjects tested tolerated the drug well, with all subjects completing the study protocol.
Based on these findings, OPI has begun preparations for a Phase 2 clinical trial in patients with hearing disorders. Several hearing disorders are being evaluated for inclusion in the study to test the efficacy of NHPN-1010, with the goal of gauging the drugs ability to promote healing and recovery of the cochlea.
About Hearing Loss
The World Health Organization (WHO) estimates that more than 360 million individuals worldwide suffer from disabling hearing loss, of which 32 million are children. The WHO also estimates that 1.1 billion people between the ages of 12 and 35 years are at risk of Noise Induced Hearing Loss (NIHL). Approximately 10% of Americans (22 million people) between ages 20 and 69 years already may have suffered permanent damage to their hearing from excessive noise exposure. This exposure can occur in the workplace, in recreational settings, and at home. NHIL is the single largest addressable cause of hearing loss problems. According to the Department of Defense and the Department of Veterans Affairs, the two most frequent service-connected disabilities in the military are related to hearing disorders, with 1.1 million Veterans living with hearing loss. Hearing loss costs the US up to $56 billion per year in lost productivity, retraining, and health care for the hard of hearing.
About Otologic Pharmaceutics, Inc.
The innovative approach of Otologic Pharmaceutics, Inc. (OPI) to hearing loss therapeutics has lead to the successful completion of single and multi-dose Phase 1 trials by OPI’s lead product, NHPN-1010, a treatment for acute sensorineural hearing loss. As a treatment for Noise Induced Hearing Loss (NIHL) and tinnitus, NHPN-1010 has shown potential effectiveness. NIHL affects a wide range of people, from the military to industry workers in multiple settings. Other OPI product candidates are being studied for use in treating chronic hearing loss through the regeneration of sensory hair cells. Hearing loss and loss of balance are commonly caused by the loss of sensory hair cells, which can result from exposure to diverse factors, including toxins, infection, trauma, or ageing. Once lost, hair cells do not spontaneously regenerate in mammals, resulting in permanent loss of hearing and balance.
About The Hough Ear Institute
The Hough Ear Institute (HEI) is a non-profit 501c3 organization dedicated to improving hearing and balance of people worldwide. Their mission is the discovery and implementation of advanced technologies, through research, education, and humanitarian efforts, to achieve the dream “that all who have ears will hear.”
Accele Venture Partners Announces Series A Financing of Otologic Pharmaceutics (OPI)
Financing to Support Entry of OPI’s Lead Product Candidate – NHPN-1010 – into Clinical Testing First Half of 2014
OKLAHOMA CITY, April 3, 2014 /PRNewswire/ — Accele Venture Partners, the investing arm of life sciences accelerator Accele Biopharma, Inc., announced today that it has co-led a $4.1 million Series A private equity financing of Otologic Pharmaceutics, Inc. (OPI), a development-stage biopharmaceutical company committed to developing and commercializing novel pharmacological solutions and approaches to treat hearing disorders. Accele Venture Partners co-led the Series A with i2E, Inc., a nationally recognized private not-for-profit corporation focused on growing innovative small businesses in Oklahoma. Other investors included the Oklahoma Life Science Fund. Proceeds of the Series A financing will be used to advance the development of OPI’s lead product candidate, NHPN-1010, for the treatment of noise-induced hearing loss (NIHL), which is expected to enter Phase 1 clinical testing in the first half of 2014.
In connection with the financing Accele Biopharma’s Chairman and CEO Clayton I. Duncan has been appointed as OPI’s CEO, replacing David Karlman in this position. Additional members of the OPI senior management team include: Richard Gammans, Ph.D., also of Accele Biopharma and recently appointed to the newly created positions of OPI’s Chief Operating Officer and Head of R&D; OPI Co-Founder and Chief Medical Officer Richard Kopke, M.D.; Company Co-Founder and Chief Science Officer Robert Floyd, Ph.D., and Kelle Jones, Vice President-Finance.
Mr. Duncan stated, “Accele is dedicated to supporting groundbreaking approaches to improve human healthcare, and we believe OPI’s programs have the potential to address substantial unmet needs for patients with hearing loss. Currently, there are no FDA-approved drugs on the market for hearing loss, and OPI’s lead product candidate, combining two well-characterized compounds, has shown promising activity in reducing hearing loss in IND-enabling preclinical studies. Applications may include hearing loss experienced by individuals in a number of occupations, such as construction and manufacturing, as well as those who have served our country in military service, in addition to hearing loss associated with cancer treatments, such as cisplatin.”
Mr. Duncan added, “I am excited to join the OPI team and also am grateful for the service of David Karlman and for the progress the Company has made in advancing its lead program toward the clinic.”
Mr. Duncan has over 20 years’ experience as a biopharmaceutical CEO, heading seven biopharmaceutical companies – Synereca Pharmaceuticals, Vindica Therapeutics, Ercole Biotech, Entegrion, Incara Pharmaceuticals Corporation, Sphinx Pharmaceuticals Corporation and CRX Medical. As a CEO, Mr. Duncan has raised over $150 million in venture and public capital markets, including two successful IPOs, and has built operational biopharmaceutical companies from initiation, negotiating seven R & D collaborations with major pharmaceutical and biotech companies and profitable exits through the sale of three biopharmaceutical companies.
Dr. Gammans has managed a broad array of product development activities, including market evaluation, clinical research, biometrics, regulatory affairs and manufacturing. He has extensive experience in supporting investor relations and business development efforts and more than 30 years’ experience in all aspects of drug R&D and scientific support of marketed products. He has contributed to the development and regulatory approval of seven new molecular entities with over 50 national marketing authorizations in Western Europe and North America, including seven approved United States NDAs.
About Hearing Loss
The World Health Organization estimates that more than 600 million individuals worldwide suffer from some form of hearing loss. Approximately 10% of Americans (22 million people) between ages 20 and 69 already may have suffered permanent damage to their hearing from excessive noise exposure. This exposure can occur in the workplace, in recreational settings, and at home. Noise-induced hearing loss is the single largest addressable cause of hearing loss problems. Cancer therapy-induced hearing loss also is a major addressable cause of hearing loss. Hearing loss costs the US up to $56 billion per year in lost productivity, retraining and health care for the hard of hearing.
About Otologic Pharmaceutics, Inc.
OPI’s lead product, HPN1010, an oral treatment for Acute Hearing Loss, is scheduled to enter clinical trials in 2014. Extensive pre-clinical research, done collaboratively through OMRF and HEI supported by nearly $ 5.4 million from the Department of Defense, has demonstrated the potential effectiveness of HPN1010 to treat Acute Noise Induced Hearing Loss (NIHL) by reducing acute damage and promoting healing and recovery of the injured cochlea. NIHL is a major cause of disability in the military and for workers in certain industry settings, including oil and gas extraction and refining, manufacturing, farming and others. OPI also plans to initiate studies on the use of HPN1010 to treat Cisplatin Induced Hearing Loss (CIHL). Cisplatin, a widely used cancer treatment, causes marked hearing loss in over 75% of the nearly 700000 patients treated each year.
See www.otologicpharmaceutic.com for additional information about HPN1010.
About Accele Biopharma, Inc.
Accele Biopharma (“Accele”) and Accele Venture Partners, a related special purpose venture fund, were formed to create a capital-efficient mechanism to identify, finance and manage groundbreaking, early-stage life science technologies that have the potential to dramatically improve human healthcare, have strong commercial promise and have the potential for generating early proof of concept data. To achieve this goal Accele has assembled an experienced management team, a group of sophisticated investors, a nationally recognized advisory board, leading research facilities and the broad scientific expertise necessary to evaluate and manage such opportunities.
Founded in 2011, Accele is located on the Oklahoma Health Sciences University Campus in Oklahoma City. For more information on Accele Biopharma, please visit www.accelebio.com.
About i2E, Inc.
With more than $40 million of investment capital under management, i2E focuses on serving companies in all phases of the business life cycle, from startups looking for their first round of capital all the way to established businesses seeking funding to expand their markets or products.
They also are helping lead new business developments into the marketplace more efficiently and more quickly while providing guidance to bring more funding to Oklahoma’s researchers and entrepreneurs.
In the past year they have launched several new initiatives that more quickly identify promising new technologies developed on state research campuses and working with new companies at the earliest stages of their development to identify a viable product and market.
The Oklahoma Bioscience Association recently became part of i2E, a development that means they will carry on the organization’s mission of supporting and enhancing the biotechnology industry in Oklahoma.
Through their proven business and venture development process, i2E turns ideas into successful enterprises.
For more information on i2E, Inc., please visit www.i2E.org.
