The Science
Our approach: to address significant patient populations with hearing-related disorders lacking ANY approved pharmacotherapy

Sensorineural hearing loss

Tinnitus
Meniere's Disease

Hearing loss related to Cochlear implant surgery
Pipeline

Tinnitus
- Auditus NHPN-1010: Tinnitus
- Auditus NHPN-1010: Hearing Preservation (Cochlear Implant)
Regenerative Medicine
- Autigen siHES1: SNHL
- Autigen siHES1#7: SNHL
- Autigen siHES1+GSK3b: SNHL
〉 Our hearing loss assets for partnering, co-development and/or out-licensing
First-in-class
AUDITUS CLINICAL ASSET (HNPN-1010)
Ph II ready – small molecule, orally bioavailable combination therapy for tinnitus, cochlear implant-related hearing loss, Meniere’s Disease, with potential in hearing loss restoration

FIRST-IN-CLASS: AUDITUS CLINICAL ASSET (HNPN-1010)
Novel therapeutic concept:
- An orally bioavailable therapeutic approach for tinnitus, acute sensorineural hearing loss (SNHL), and potential for chronic SNHL
- Breaks a cycle of long-term hair cell and neuronal degeneration occurring long after injury
- Comprehensive preclinical package
- Safe and robust clinical efficacy data-in-hand
First-in-class
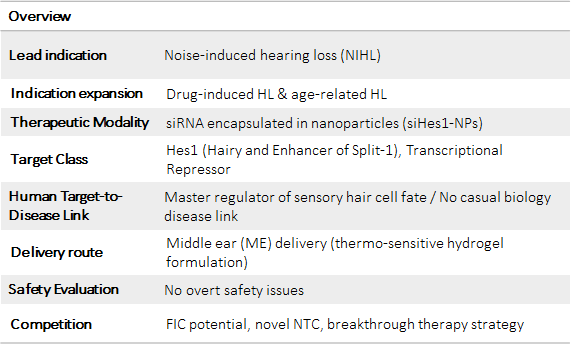
Autigen (siHes1)
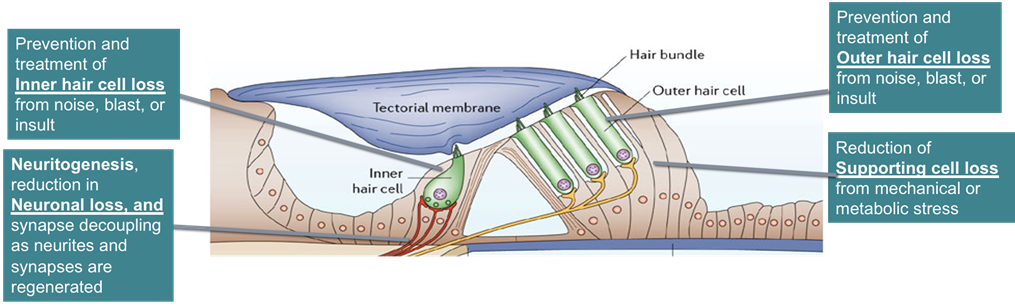
Preclinical asset: Sensory hair cell loss is core pathological hallmark of SNHL. Following acoustic trauma, Notch signaling plays an important role in initiating and resolving immune response in the inner ear. Our working hypothesis: we propose to use a siRNA [siHes1] that selectively interacts and reduces Hes1 mRNA, in turn decreasing HES1 protein levels in the supporting cells of the cochlea. This results in disinhibition of Atoh1 expression, proliferation of supporting cells (SCs), and in turn increased trans-differentiation of supporting cells into functional sensory hair cells. Ultimately, providing a functional improvement to patients suffering from hearing impairment.

Autigen: preclinical program
siRNA-mediated depletion of HES1, leads to the induction of ATOH1 expression, which is critical for hair cell differentiation, new functional inner and outer hair cells are produced.
Functional hearing recovery
Regeneration of Inner hair cells
by siRNA treatment
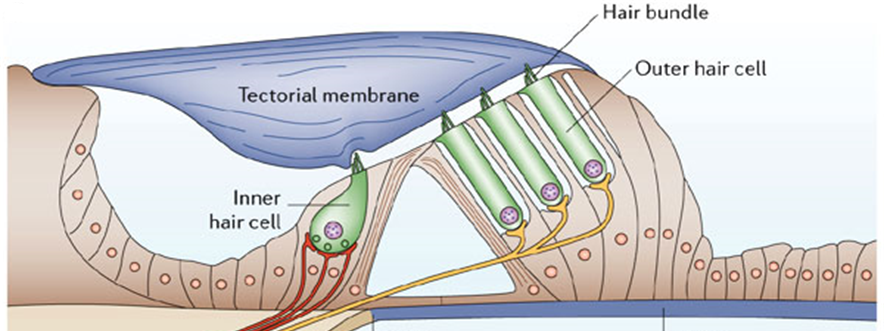
Regeneration of Outer hair cells by siRNA treatment
Induced proliferation of Supporting cells enhances siRNA regeneration efficacy
